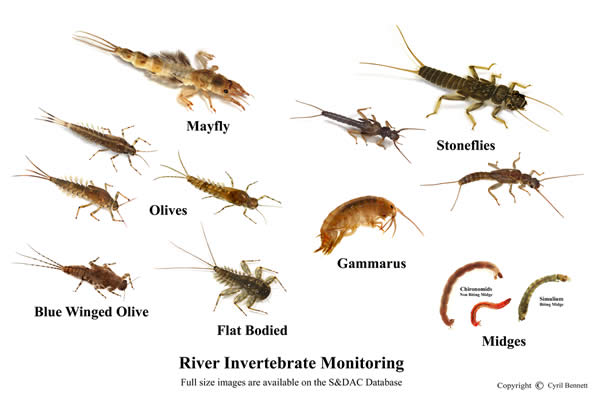
River Invertebrate Monitoring (RIM)
Sampling Method
The sampling method is the standard 3 minute kick/sweep sample using a standard pond (hand) net.
1. The different habitats within the sampling area are identified, for example, fast moving (riffles), slow water (pools), bankside (tree roots etc) and weed-beds.
2. A total sampling time of three minutes is split
proportionally between the number of habitats
identified for sampling. So, for example, if
riffles occupy 50% of the site they should be
sampled for 1½ minutes and if weeds occupy
25% of the site they should be sampled for 45
seconds.
3. The pond net is rested on the riverbed and the
area immediately upstream disturbed
(“kicked”) using the foot such that
invertebrates are carried into the net by the
current; further samples are taken moving
across and/or upstream. For sampling in weed
areas the net is “swept” through the weed-bed.
4. A further 1 minute hand search is then carried out on the rocks, woody debris etc.

Kick Sampling using standard pond net.
“Washing” the sample:
To ease the counting process it’s important to remove as much of the unwanted debris from the sample as possible without losing any of the required invertebrates. This is achieved by first tipping the contents of the net into a large bucket of river water; which is then “strained” back through the net whilst agitating the stones & gravel to dislodge all of the invertebrates; this will leave behind the unwanted heavy material of the sample. This is repeated until all of the invertebrates have been dislodged from the stones and gravel in the bucket. After a quick check is made that no invertebrates remain (i.e. heavy cased caddis larvae) the stones and gravel can be discarded from the bucket
Holding the net into the current and moving the material around in the net will then remove most of the unwanted fine silt through the mesh; some of the plant material can be removed after ensuring that any invertebrates have been dislodged. The remaining sample can then be returned to the bucket, half filled with clean water and the sample is ready to be sorted.
Identifying and Sorting the Target Groups
Sorting the sample:
The invertebrates should now be much easier to find
providing that only small “sub-samples” are taken
from the bucket (using a small aquarium net) at a
time and placed into a shallow white tray half filled
with clean water; if a large enough tray is used all (or
most) of the sample can be processed in one go. The
required invertebrates can then easily be picked out
using a pipette (turkey baster) and transferred into
some form of divided tray ready for counting.

Recording the Data
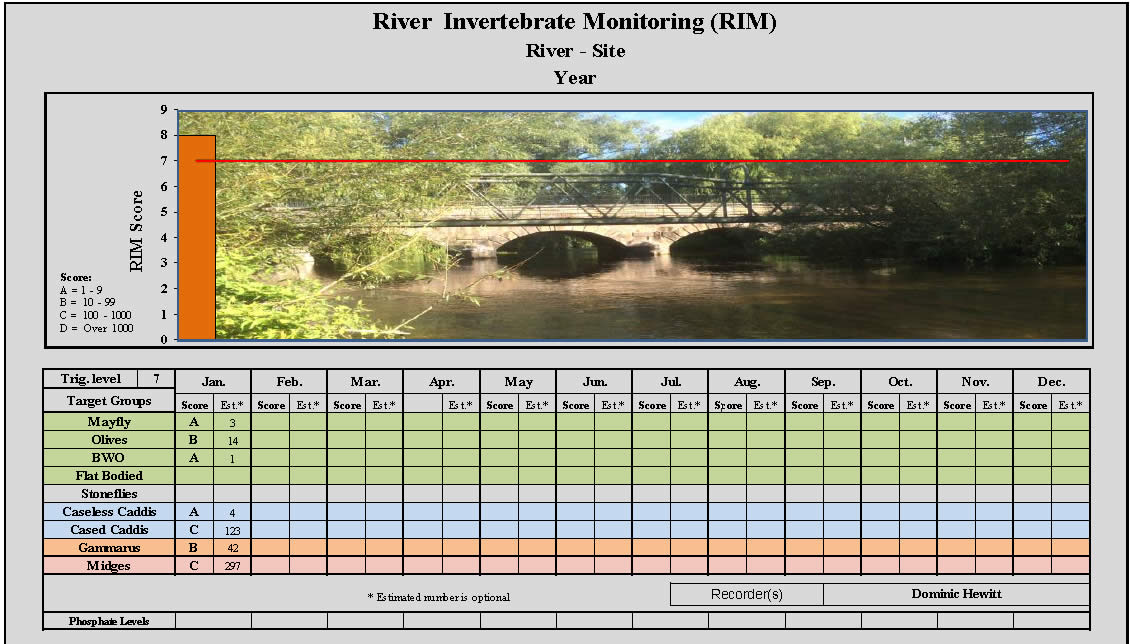
Recording Chart
Data can then be recorded onto a RIM monitoring chart (DOWNLOAD HERE) using the following abundance estimates:
(1 – 9 = A) ( 10 – 99 = B) (100 – 999 = C) ( Over 1000 = D)
(‘Midges’ use negative scores)
The Pollution Trigger level will need to be agreed with the local EA.
Full monitoring equipment can be obtained from NHBS Ltd.
Dr.Cyril Bennett MBE
Salisbury & District Angling Club.